MRI Strategies at Stanford Medicine
Body MRI Research (BMR)
According to a 2019 report by the CDC, breast cancer (BRCA) is second only to lung cancer as the most-deadly cancer for American women.1
Top 10 Cancers by Rate of Cancer Deaths
Rate per 100,000 women, United States, 2020, all races and ethnicities
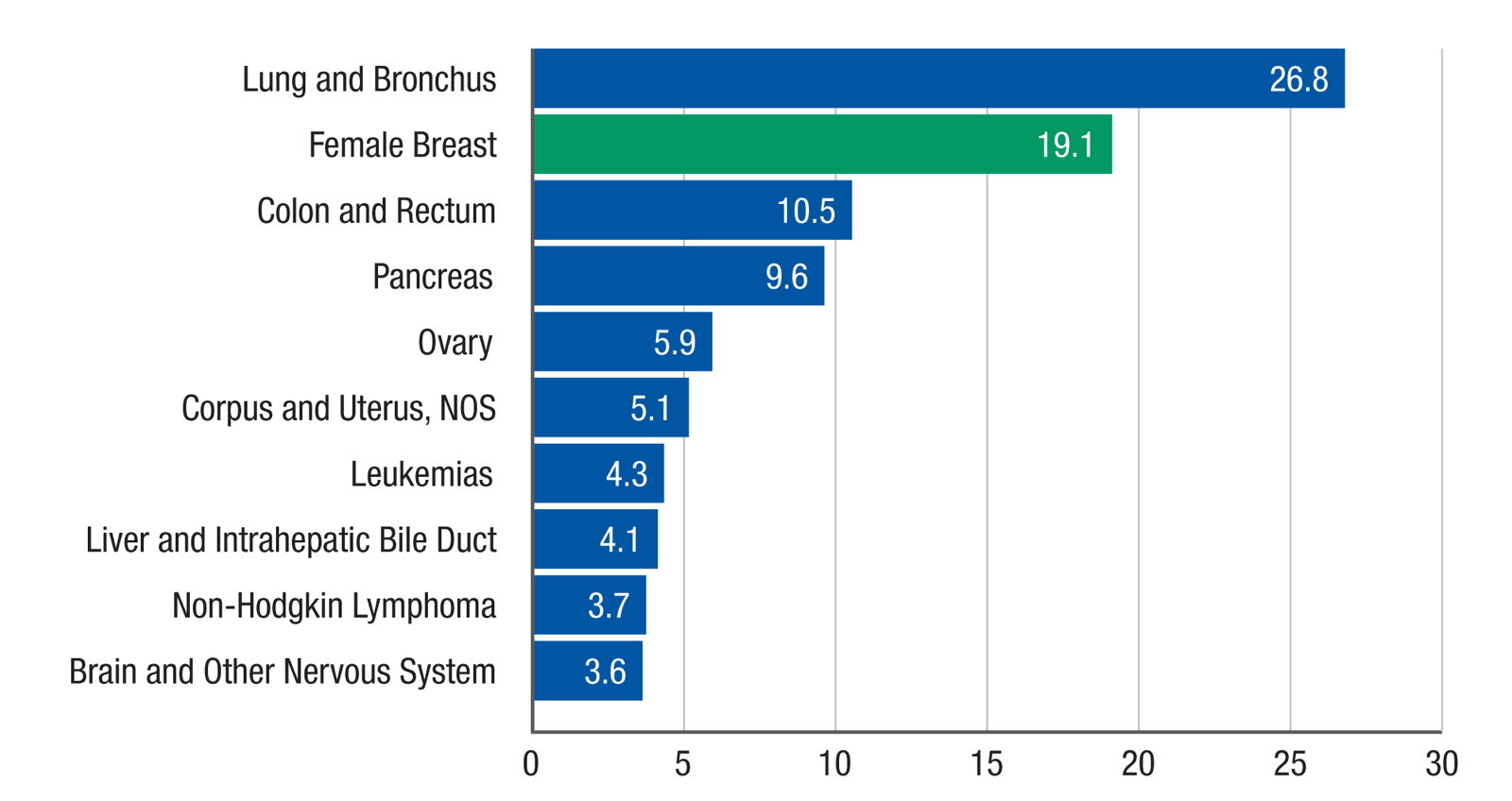
Source: U.S. Cancer Statistics Working Group. U.S. Cancer Statistics Data Visualizations Tool, based on 2022 submission data (1999-2020): U.S. Department of Health and Human Services, Centers for Disease Control and Prevention and National Cancer Institute; https://www.cdc.gov/cancer/dataviz, released in June 2023.
While the most common screening method for BRCA remains X-ray mammography, according to Stanford Medicine Body MRI Research Group (BMR), the use of MRI has much greater sensitivity for identifying breast cancers, especially among those who remain at higher risk.2
The Stanford approach to breast MRI research includes imaging at higher spatial resolution, “to better depict tumor features as well as enabling different image contrast mechanisms to help to classify malignancy and tumor sub-types.”2
The need, says Stanford, is for more advanced methods in order to generate images quickly enough and with high image resolution.
Stanford’s breast cancer assessment methods also include T2-weighted imaging and diffusion-weighted imaging (DWI). In addition, the research facility is exploring faster and sharper techniques to capture breast cancer images.
For example, while T2 and DWI are currently not as accurate for cancer detection, these methods don’t involve contrast agent injections, so they may reduce costs and provide wider access to MRI for more women.2
New U.S. Food and Drug Administration Ruling on Informing Women About Breast Density
In a move designed to help detect more breast cancer cases and in earlier stages, on March 9, 2023, the Food and Drug Administration (FDA) issued a new ruling requiring breast imaging centers to inform women about their breast density and the associated risks of developing cancer, since dense breast tissue is a known factor that not only increases cancer risk but makes it harder to detect malignancies on a mammogram.3,4
In Ursin et al.,5 it was shown that breast tumors are more likely to develop in the tissue where mammograms indicate the highest breast density, versus the less dense, more fatty areas of the breast.
The new FDA ruling doesn’t specifically recommend further screening with MRI for women with dense breasts. That’s consistent with the American Cancer Society (ACS) Guidelines for Breast Screening with MRI as an Adjunct to Mammography, which recommend MRI for women with a 20-25% higher risk.
The guideline says women with “extremely dense breasts on mammography” are among the subgroups of women for which “the available data are insufficient to recommend for or against [additional] screening.”3
Nevertheless, the FDA says the conversation on dense breasts must be had with each patient.
The individuals who appear are for illustrative purposes. All persons depicted are models and not real patients or healthcare professionals
Women with extremely dense breast tissue and no other risk factors probably hit the 20 percent risk marker based on density alone, says Wendie Berg, a radiology professor at the University of Pittsburgh School of Medicine.6 Women whose breasts are dense but not in the highest category “probably [have] a lifetime risk of more than 20 percent” if they have other cancer-causing risk factors, Berg says.6
MRI combined with mammography is generally the most effective screening protocol for women with dense breasts because MRI is highly accurate and results in fewer false positives than ultrasound, Berg says.6
More Dialogue Needed
Is the general public aware that the density of breast tissue is a cancer risk factor?
A recent qualitative study says “no.”
Among more than 2,300 women aged 40-76, most participants in the survey did not associate having dense breasts with a higher cancer risk.7 Clearly, more awareness is necessary, and can help to make a difference in detection and ultimately, survival rates.
References:
- Centers for Disease Control and Prevention. United States Cancer Statistics: Data Visualizations. https://gis.cdc.gov/Cancer/USCS/#/AtAGlance/. Accessed June 23, 2023.
- Stanford Medicine Body MRI Research Group (BMR). Breast MRI. https://med.stanford.edu/bmrgroup/Research/BreastMRI.html. Accessed May 15, 2023.
- Saslow D, Boetes C, Burke W, et al. American Cancer Society Guidelines for Breast Screening with MRI as an Adjunct to Mammography. CA Cancer J Clin. 2007 Mar-Apr;57(2):75–89.
- U.S. Food and Drug Administration. FDA Updates Mammography Regulations to Require Reporting of Breast Density Information and Enhance Facility Oversight. https://www.fda.gov/news-events/press-announcements/fda-updates-mammography-regulations-require-reporting-breast-density-information-and-enhance. Accessed May 15, 2023.
- Ursin G, Hovanessian-Larsen L, Parisky YR, et al. Greatly increased occurrence of breast cancers in areas of mammographically dense tissue. Breast Cancer Res. 2005 Jun;7(5):R605-8.
- Small L. (2023, Apr 10). What the FDA Ruling about ‘Dense Breasts’ Means for Cancer Risk and Screening. Scientific American. https://www.scientificamerican.com/article/what-the-fda-ruling-about-dense-breasts-means-for-cancer-risk-and-screening/.
- Beidler LB, Kressin NR, Wormwood JB, et al. Perceptions of Breast Cancer Risks Among Women Receiving Mammograph Screening. JAMA Netw Open. 2023 Jan;6(1):e2252209.

